How to Build Pocket Crossed Filters for Gemstone Testing
The crossed filters technique allows gemologists to detect chromium in gems. Learn how to build a portable device for conducting this useful ID test.
4 Minute Read
What is the Crossed Filters Gem Testing Technique?
With crossed filters, gemologists can determine the presence of chromium in gemstones, most notably ruby, red spinel, emerald, pink topaz, and alexandrite. The crossed filters technique (not to be confused with the polarized crossed filters technique) is based on the emission of white light filtered through a blue medium. This filtered light, now blue, illuminates a gemstone observed through a red filter. Gemologists conduct this procedure in darkness.
If the gems observed through the crossed filters technique contain chromium, they will fluoresce strongly. For example, a ruby will look like a piece of burning coal. However, iron in the gemstones may significantly decrease or completely eliminate the effect. Thus, a synthetic ruby without any iron content will exhibit this effect quite intensely.
A Brief History of Crossed Filters
Sir George Gabriel Stokes (1819-1903) pioneered this testing technique, but not for gemology. Rather, he used it for the determination of weak fluorescent samples. In 1959, Sir B.W. Anderson adopted a similar technique, based on Stokes's observations, for gemological use.
Anderson utilized a 500 cc glass lab balloon, filled with a saturated solution of copper(II) sulphate pentahydrate (CuSO4·5H2O) in distilled water. A strong incandescent lamp (500 W) illuminated the balloon, placed in an isolated and cooled box. Then, the filtered light (blue after passing through the solution) hit the gemstones. They were placed over a black support and observed through a red gelatine photographic filter. You can see the general idea illustrated in Figure 1 at the top of this article.
In their 1993 study of the crossed filters method, D.B. Hoover and A.F. Theisen showed that the predominant chrome excitation in chromium-bearing gems occurs in a blue band centered near the 440 nm absorption line.
Interested gemologists can find several applications for this gem testing technique. However, for this article, our main objective is to turn that cumbersome device in Figure 1 into a reliable, portable crossed filters system.
How to Make a Pocket LED Crossed Filters Device
New LED (light-emitting diode) technologies make very simple and inexpensive sources of light, so gemologists should have these for their labs.
The Blue Filter/Light
For our pocket crossed filters, buy an inexpensive penlight, one that uses two AAA or AA alkaline batteries, and a strong blue LED. You can find these at any electronic parts store or online. Replace the penlight bulb or white LED with the blue one. To make sure you connect the blue LED correctly, see Figure 2
The Red Filter
For the red filter, you could use a sheet of red plastic or a red photo filter. However, you have another option: a red plastic filter from a barcode reader. These devices, used in shops everywhere, have red plastic filters for the incoming/outgoing red laser. When the readers break, they usually get trashed rather than repaired. Try to find one and remove the plastic filter. However, please note that this plastic is a special acrylic glass. Don't cut, drill, or flame it because it will crack. You should use it as is.
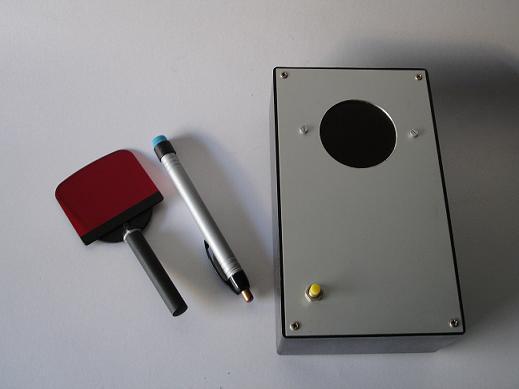
In Figure 3, you can see our adaptation. From left to right, we have the red filter with an attached handle, the modified penlight, and a special box. This optional box setup contains five blue LED strips and a circular opening on the top with a red filter underneath. On the side (not shown), it has another circular opening for introducing a gemstone or small jewelry piece for testing. The interior is spray painted black. (I've added an on/off switch for the LED lights).
How to Make a Copper Sulphate Crossed Filters System
The LED adaptation makes a very effective and portable crossed filters device. However, LED and copper sulphate may produce fluorescent effects of different magnitudes. Well, if you want the best of both worlds, you can also create a copper sulphate crossed filters system. See the differences for yourself.
First, find two transparent gemstone boxes, one big and one small. Glue the bottom and the cover of the small box together using some epoxy glue. Make sure that no air or liquid will escape.
Prepare the copper sulphate saturated solution. Do it warm. When it cools, filter it well and add one drop of sulfuric acid to stabilize it. (Carefully follow all safety procedures for using sulfuric acid).
Next, flame a nail and make a hole in the lateral side of the small box. With a syringe, inject the liquid inside the box. Do it slowly, letting the bubbles come up as the box fills. When done, close the hole using a generous layer of the epoxy glue. Also, at any lateral face of the box, glue a rod or L-shaped support that allows you to fix the box to some support. Cover the rear and the front of the box, then paint the lateral faces of the box black.
Use the larger box to hold the gem. For a red filter, use one from the LED setup. Now, you have a good copper sulphate crossed filters system.
Using a Copper Sulphate Filter with a Spectroscope
You can also use your copper sulphate filter for spectroscopy observations. Figure 5 shows how you can use it with a spectroscope.
This method is quite useful. For example, look at Figure 6. Guess how to separate a natural red spinel from a synthetic one.
Dr. Raul Berenguel, PhD.
Dr. Raul Berenguel holds degrees in History (Scientific Branch, 2008) and Contemporary Art (PhD, 2012) from the Universidade Aberta. He has specific expertise in the fields of history, gemology, and contemporary art. In addition, he’s also an expert in applying traditional gemological techniques to investigating art objects made from gem materials.
Related Articles
Gemstone Refractometer Mini-Quiz
Refractometer Guide, Part 1: Refractive Index Testing
Dichroscope Guide for Gemologists
Gemology Book Reviews: Careers
Latest Articles
Quartz Toxicity: Understanding the Risks for Jewelers and Wearers
Synthetic Amethyst: What is it and How is it Made?
Hambergite Value, Price, and Jewelry Information
Pearl Simulants: How to Spot Faux Pearls
Never Stop Learning
When you join the IGS community, you get trusted diamond & gemstone information when you need it.
Get Gemology Insights
Get started with the International Gem Society’s free guide to gemstone identification. Join our weekly newsletter & get a free copy of the Gem ID Checklist!