Understanding Gem Synthetics, Treatments, And Imitations, Part 2: Crystal Growth
Learn about the different crystal growth methods used to artificially create gemstones in laboratories.
16 Minute Read
Editor's Note: Gemstones can be artificially created in laboratories using any one of several possible crystal growth methods. This five-part series of articles, "Understanding Gem Synthetics, Treatments, and Imitations," is a chapter from Dr. Joel Arem's forthcoming book, Gems and Jewelry, 3rd Edition. © Joel E. Arem 2011-2013. The International Gem Society (IGS) gratefully thanks Dr. Arem for his contributions to the field of gemology and for allowing us to reproduce this chapter.
A Bit of History
Glass has been manufactured for thousands of years. Glassmaking was considered a great art by the ancient Egyptians, and Greek and Roman jewelry studded with glass replicas of gems can be seen in museums. Even today glass is a widely used and popular substitute for colored gems such as ruby, emerald, aquamarine, and amethyst and it can sometimes be effective and attractive. Glass stones are often set with a backing of metallic foil. The foil reflects light and creates a far greater brilliance than the glass alone could achieve. But glass lacks the hardness and dispersion of many natural gemstones, and mankind has long sought better gem substitutes.
One by one, during the past hundred years, each of the major gems has been duplicated in the laboratory. The first to appear were ruby and sapphire, followed by spinel, quartz, emerald, diamond, opal, turquoise, and chrysoberyl. These "synthetic gems" are homocreates* and are therefore optically and chemically identical with their natural counterparts. The long list of gemstone synthetics now includes aquamarine, golden and red beryl, garnet, zircon, opal, turquoise, and many others.
In recent years technological developments in the areas of semiconductors and lasers have required the development of new and special crystals with useful optical or electronic properties. Some of them are brightly colored or have other characteristics suitable for use in jewelry. These new synthetic gems have no natural counterparts. They are laboratory creations that have extended the world of gemstones in new and unique directions.
It is important to remember that even imitation materials can be so good at simulating natural gems that the eye alone cannot tell the difference. A safe generalization is that, with few exceptions, the authenticity and origin of a gem cannot be determined with the naked eye. Color is not a suitable criterion, because nearly any color can be duplicated with the right combination of chemicals. Synthetics can so resemble natural gems that even gemologists are sometimes fooled. Manufacturers may even try to purposely add natural-looking inclusions and imperfections to their products. The production of "gem" materials has become a major business, and manufacturing techniques have become a fine art. Detection of synthetics is an ongoing challenge, and should be entrusted only to a professional gemologist or gem laboratory. Proper identification often requires expensive and sophisticated scientific equipment that is far beyond the reach of a typical jewelry store. The jeweler who might "authenticate" a stone by squinting at it against a sunlit window is often fooling both himself and his client.
There is nothing intrinsically wrong with synthetic gems. They make the colors and brilliance of the finest gemstones affordable to a vast portion of the gem-loving marketplace. But the reasons for acquiring synthetic versus natural gems are often very different, and problems arise only when a synthetic or treated material is sold as a natural stone.
For example, a five-carat ruby of the finest color and transparency might cost $100,000 per carat, or more. A synthetic ruby of identical color and clarity that might, to the eye, be indistinguishable from the natural stone, could sell for a few hundred dollars, or less. The natural gem has tremendous value because of its scarcity. But to the person who simply wants a ruby for personal adornment because of its rich color and brilliance, the synthetic might be perfectly suitable and should not be downgraded because of its low cost and "ignoble" origin.
Crystal Growth
A crystal is characterized by long-range order; that is, the atoms in a crystal are arranged in regular, periodic arrays or patterns (like wallpaper). The object of crystal growth is to add more atoms and perpetuate the pattern. A seed crystal is used to provide the basic template, and the raw material (loose atoms) remains mobile by being vaporized, melted, or dissolved in a solution. Thus, we may speak of vapor growth, melt growth, flux growth, or solution growth, depending on the medium used for crystallization. Crystal growth is achieved by forcing the unattached atoms in the growth medium to attach themselves to the seed. This is theoretically relatively simple to do. All that is required is to cause the growth medium to contain more unattached atoms than the medium can handle at a specific temperature. Unfortunately, it is not so easy to make the atoms go exactly where you want them to go. This is why some people speak of the "art and science of crystal growing."
In human societies, when cities become too crowded there is often an exodus to the suburbs. If a growth medium, let's say a solution, is forced to contain excess dissolved material at a given temperature, the system may turn out to be "out of equilibrium" at a lower temperature. The direction of spontaneous change is the one that "dumps" some of the dissolved material back out of solution, like commuters fleeing the crowded downtown in favor of the quiet countryside! If the "dumping tendency" is strong enough (for example, a drop in temperature) the atoms will stick together and create many small clusters, called nuclei. The alternative to random, uncontrolled nucleation is to provide a template, or seed crystal, for the "dumped" atoms to attach to.
Crystal growth is tricky and many things can go wrong. In light of this, it is absolutely amazing that gems exist.
A gemstone is a transparent and outwardly perfect crystalline mass, (ideally) free of visible imperfections or flaws, of uniform color and sometimes of immense size. It is difficult enough to grow such perfect crystals in a controlled laboratory environment. It is nothing short of miraculous that, given the randomness of natural environments, there exist crystals large and perfect enough to yield gemstones.
Following is an abbreviated summary of the basic methods used to grow crystals. All of the gemstones being made in laboratories are made by one or more of these methods.
Vapor Growth
Substances best grown from vapor are those that pass directly from a solid to a vapor when heated or those whose components can easily be transported in vapor form. Materials that pass readily from solid to vapor are said to be volatile. In vapor-transport techniques, the desired substance reacts (usually at a high temperature) with another material, and the products of the reaction are even more volatile than the original substances. These newly formed products are moved to a new location, usually at a lower temperature, where they react in a reverse way to recreate the starting materials. If the procedure is done carefully, the reaction yields single crystals. Vapor-grown crystals are characteristically long needles or thin plates; in some cases crystal growth yields lacelike aggregates known as dendrites (for example, snowflakes).
CVD
Chemical Vapor Deposition is a technique that has been used for decades to put thin coatings onto surfaces. The most familiar is the blue coating on camera and binocular lenses. Its only significant gemological application is in growing diamond (to be discussed later).
Melt Growth
Most natural crystals were formed in molten environments deep within the Earth. The sizes of the crystals (grains) in a rock and the way in which the grains have grown together are meaningful to geologists and tell a great deal about the cooling history of the rock. Gemstones, including olivine (peridot), feldspar, and others, are occasionally cut from larger crystals that are found in such igneous materials.
The general term for melt growth is solidification. Everyone grows crystals from a melt. Water, after all, is nothing more than molten ice, a crystalline solid that freezes (solidifies) at only 32°F. Snowflakes, although dendrites, are single crystals of ice. However, the ice cubes in your refrigerator are not. Uncontrolled freezing of a melt generally results in the formation of many tiny crystallites that all grow at the same general rate to fill up the available space. An ice cube is thus a polycrystalline aggregate, consisting of myriad inter-grown crystals. Poured ingots of molten metals crystallize in much the same way.
Growth from the melt is very convenient and in many cases requires relatively unsophisticated equipment. This method is unsuitable, however, for growing materials that contain water or volatile components; such materials decompose at their melting point. In technical language, a "congruently melting" material is one that does not change composition at the boundary between the solid and liquid state and can therefore be grown by one of the following methods.
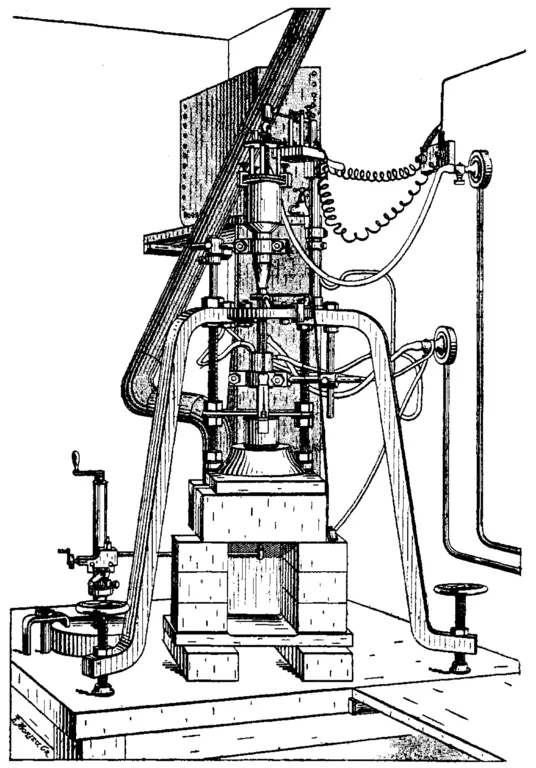
The Verneuil Technique, or flame fusion, was developed in the late 1800s by August Verneuil, one of the great pioneers of gemstone synthesis. Verneuil had deposited sealed papers with the Paris Academy of Sciences in 1891 and 1892. When opened in 1910, these documents revealed the details of Verneuil's work on ruby synthesis, opening the door to large-scale production. The equipment detailed by Verneuil was so cleverly designed that modern factories still employ furnaces with essentially the same specifications as the original. Perhaps several hundred materials have been grown by the Verneuil method, and it is one of the least costly of all crystal growth techniques. Verneuil crystals are routinely sold for only pennies per carat and are readily available to hobbyists and gem cutters.
The primary feature of a Verneuil furnace is an oxy-hydrogen or oxy-acetylene torch. A powder of the substance to be grown is dribbled through this flame, and the molten drops fall onto a rotating rod, which is slowly withdrawn. The withdrawal rate is adjusted carefully, so that the molten droplets "raining" onto the rod solidify in a controlled fashion and build up a single crystal. The purity of the finished crystal is a function of the starting powder and the atmosphere in which the crystal is grown. The quality of the Verneuil crystal, or boule (French for ball) depends on the purity and particle size of the feed powder, the flame temperature, rate of rotation and withdrawal of the seed rod, and the ability to shield the crystal from drafts. The popularity of the Verneuil method for crystal production is illustrated by the fact that, by the 1920s, factories in Europe were turning out hundreds of millions of carats of Verneuil crystals annually. Among the gem materials produced commercially in this way are sapphire, ruby, star corundum, spinel, rutile, strontium titanate, and a vast array of oxides and other compounds. NOTE: a ruby in a ring inherited from your great-grandmother does not have to be natural just because it's over 100 years old!
The Czochralski technique, or "crystal pulling" was originally developed to measure the speed of crystallization of metals. It is now as important as the Verneuil method in gemstone crystal growth. The technique involves the melting of a starting powder in a crucible, generally platinum, iridium, graphite, or ceramic. A rotating rod with a tiny seed crystal on the end is lowered into the crucible until it just touches the melt and then is slowly withdrawn. Crystallization at the interface between the melt and the seed proceeds in two ways: 1. Surface tension pulls some of the melt slightly out of the crucible onto the seed. Once this material leaves the melt, it cools just enough to solidify, adding to the seed crystal. 2. Heat conduction allows the solid to extend very slightly into the melt, assuring that ample material is pulled out to make the growing crystal ever larger. Crystal growth continues in this way until the entire contents of the crucible have been pulled out and added to the rod.
The pull-rate is normally on the order of 1 mm to 10 cm per hour. Czochralski crystals can be enormous - the size of baseball bats! A number of technologically vital crystals, such as pure silicon, are grown by pulling, as are many materials that are cut as gems. These include ruby, sapphire, YAG, GGG, alexandrite, and a wide variety of unusual oxides.
The Bridgman-Stockbarger Method was developed around the same time by R. W. Bridgman (American), D. C. Stockbarger (German), and the Russians J. Obreimov, G. Tammann, and L. Shubnikov in the period 1924-1936. A specially shaped container is used, generally a cylindrical tube that tapers to a cone with a small point at one end. The tube is filled with powder of the desired crystalline material and lowered through a heater (radio-frequency or electrical resistance types are most common), pointed side down. The material in the tube melts, but the small conical tip is the first part of the container to emerge from the heater. In ideal circumstances (not all that difficult to achieve) the first bit of molten material to solidify forms a single crystal, rather than a polycrystalline aggregate. Further solidification continues as an extension of the pattern provided by this induced seed crystal until the entire cylinder is frozen and the container is filled with a single crystal.
There are many variations of this technique, some adapted for specialized applications such as the growth of high-purity metals. The method is extremely simple in concept and can be employed to grow truly immense crystals, the largest to date being more than three feet across and weighing more than a ton (sodium iodide, cesium iodide, and others). It is commonly used for the growth of halides, many sulfides, and a variety of oxides.
A problem arises when materials are so reactive that they cannot be melted, even in such un-reactive containers as platinum and iridium, or if the melting point of the material to be grown exceeds that of the available container materials. The latter is the case with cubic zirconium oxide (CZ) which melts at the fantastically high temperature of 2750°C. (4982°F.) Single crystal growth of CZ was not managed until the 1970s, when a research group in the USSR perfected a technique (previously known) called skull melting. The "skull" is an open-ended cup made of copper cylinders, filled with powdered zirconium oxide, and heated until the powder melts. The cylinders are hollow and water cooled, so the molten zirconia is thus effectively contained within a 1-mm thick shell of solid zirconium oxide that forms just inside the copper walls. The entire assembly is then allowed to slowly cool until the entire mass has solidified. A typical skull contains about a kilogram of material, of which half emerges as cuttable CZ. Zirconium oxide is the only important gemstone material grown by this method and it is made in a wide variety of colors and in many different locations. Global CZ production is reported in tons, rather than carats!
Solution Growth
Solutions are perhaps the most familiar crystal growth environments. Even the simple act of making a cup of instant coffee is a study in solubility. If you go swimming at the beach, the slippery and often uncomfortable feeling you get after a while is caused by evaporating seawater leaving a fine crust of sodium chloride and other salts on your skin. You can even see their crystal shapes (cubes in the case of sodium chloride) with a magnifying glass.
Solution growth has major advantages, including high mobility of dissolved components, convenience, and ease of control. The apparatus for solution growth can be as simple and inexpensive as a pot of water and some mason jars; most gemstones, however, require far more elaborate and expensive apparatus!
Although as much as five pounds of sugar can be dissolved in a quart of boiling water (you will find out about this if you make hummingbird food), such high solubilities cannot be found among oxides and silicates. In addition, although pure water is an excellent solvent for many compounds, the materials of gemological interest have such low solubilities that, for practical purposes, they may be considered insoluble. As in the case of natural environments, however, a bit of mineralizer (for example, sodium hydroxide) dissolved in hot water dramatically increases its capability for dissolving silicates such as quartz, beryl, etc. It is also much more effective to put the water under both high pressure and high temperature. Under these conditions, called hydrothermal growth, many mineral crystals can be duplicated in the laboratory. Moreover, since these are the same kinds of conditions that prevail in the ground, the resulting crystals often look strikingly like those found in ore deposits.
A major difference, however, is size. Nature is relatively unconcerned about the corrosion of container walls, the rupturing of growth vessels if the pressure gets too high, or even the exact chemistry (or purity) of the growth solutions. Nature produces very high temperatures and pressures with impunity. The result can be spectacular indeed: spodumene crystals up to 40 feet long, feldspars the size of railroad boxcars, and people-sized quartz crystals. To date the largest hydrothermal (quartz) crystals grown in laboratories weigh less than a few hundred pounds. The growth of sugar crystals (rock candy) and other salts can be achieved at room temperature and pressure in simple containers. Silicates cannot be grown in this way. These substances can, however, be crystallized in steel cylinders called bombs, which are loaded with feed material, water, mineralizers, and seed crystals, and placed inside a sealed unit called an autoclave.
Hydrothermal growth apparatus is a pressure cooker. The bomb is heated within the device, and, since it is sealed, once the water in it expands to fill the cylinder, the pressure rises as the temperature is increased. The temperature is carefully monitored, and the water added to the bomb is exactly measured, to achieve a predetermined pressure level. Mistakes here, not surprisingly, can be embarrassing!
Hydrothermal synthesis is not of great significance for technological applications, except in the case of quartz. It is, however, of tremendous importance for synthetic gemstones because so many natural materials form hydrothermally within the Earth. Among the gems routinely produced in this way are emerald, amethyst, and citrine. Hydrothermal growth is especially suited to materials that contain water or other volatile components and that therefore decompose on melting.
Flux Growth
Water is molten ice, and is an effective solvent for many substances familiar to us all. It is not, however, a powerful enough solvent to dissolve most oxides, silicates, and other hard materials. Ice is a crystalline solid that melts at 32°F. Other crystalline solids can be melted at temperatures as low as a few hundred degrees. If water (molten ice) is a good solvent, what about the solution capabilities of other molten substances? It turns out that a number of compounds, including borax, lithium oxide and molybdenum oxide, potassium fluoride, lead oxide and fluoride, and other mixtures, are powerful solvents when melted; in fact, some crystal growers believe that it should be theoretically possible to find a molten-salt solvent for any given crystal. The earliest gem crystals, the rubies made by [Edmund] Fremy, were grown from molten-salt solutions of corundum. A vast array of compounds, many of gemological interest, can be grown in this way, including alexandrite and emerald.
* A Note From Donald Clark: Dr. Arem's article, "Understanding Gem Synthetics, Treatments, and Imitations," is a wonderful piece. I have a great deal of respect for Dr. Arem. His Color Encyclopedia of Gemstones is the best reference of its type. He once helped me with a difficult identification. I didn't expect a personal letter from him and was pleased that he would go out of his way to help me. This was before the existence of the IGS. However, you should be aware that he defines the words "synthetic" and "homocreate" in a manner inconsistent with our industry standards. In my article, "How Gems Are Classified," I define "synthetic" as "materials that duplicate their natural counterparts" and "homocreate" as materials that have "no counterpart in nature," in accordance with the Gemological Institute of America (GIA).
Understanding Gem Synthetics, Treatments, And Imitations, Part 1: An Introduction
Understanding Gem Synthetics, Treatments, And Imitations, Part 2: Crystal Growth
Understanding Gem Synthetics, Treatments, And Imitations, Part 3: Synthetic Diamond
Understanding Gem Synthetics, Treatments, And Imitations, Part 4: Synthetic Gemstone Guide
Understanding Gem Synthetics, Treatments, And Imitations, Part 5: Identifying Gemstone Treatments
Joel E. Arem, Ph.D., FGA
Dr. Joel E. Arem has more than 60 years of experience in the world of gems and minerals. After obtaining his Ph.D. in Mineralogy from Harvard University, he has published numerous books that are still among the most widely used references and guidebooks on crystals, gems and minerals in the world.
Co-founder and President of numerous organizations, Dr. Arem has enjoyed a lifelong career in mineralogy and gemology. He has been a Smithsonian scientist and Curator, a consultant to many well-known companies and institutions, and a prolific author and speaker. Although his main activities have been as a gem cutter and dealer, his focus has always been education. joelarem.com
Related Articles
Build Your Own Gemology Tools
Gem Formation: How are Gemstones Created?
Guide to Organizing Your Gemstone Collection
Synthetic Gemstone Inclusions
Latest Articles
Brazilianite Value, Price, and Jewelry Information
Ruby-Glass Composites vs Leaded Glass Clarity Enhancements
Morganite Buying Guide
How Do Amethysts Form?
Never Stop Learning
When you join the IGS community, you get trusted diamond & gemstone information when you need it.
Get Gemology Insights
Get started with the International Gem Society’s free guide to gemstone identification. Join our weekly newsletter & get a free copy of the Gem ID Checklist!